 |
| Click for full size images |
NanoViricides Signs a Non-Disclosure Agreement with the Lovelace Respiratory Research Institute for IND-enabling Efficacy Studies on FluCide® and for Testing its Novel Drug Candidates against the Highly Lethal MERS Human Coronavirus - Excerpts:
Mon, Aug 5, 2013 7:00 AM EDT
WEST HAVEN, Conn.--(BUSINESS WIRE)--
NanoViricides, Inc. (OTC BB: NNVC) (the "Company") said it has signed a non-disclosure agreement (NDA) with the Lovelace Respiratory Research Institute (“LRRI”). The Company intends to enter into a Master Services Agreement with LRRI for the IND-enabling efficacy studies of both its broad-spectrum injectable and oral FluCide® drug candidates. These studies will employ multiple unrelated subtypes and strains of Influenza A, including the novel H7N9 strain, the subtype which is currently circulating in China. The Company has already shown that the injectable and oral FluCide drug candidates are substantially more effective than oseltamivir (Tamiflu®) in controlling influenza A virus infections in a highly lethal animal model using two unrelated subtypes of influenza A, namely H1N1 and H3N2. In addition to FluCide, LRRI will also be able to evaluate the Company’s anti-MERS drug candidates in cell culture and animal models when available. The NDA enables the scientists at the Company and LRRI to exchange confidential and proprietary information in preparation for the intended studies.
The injectable FluCide drug is intended for severely ill hospitalized patients while the follow-on oral drug is intended for use in out-patients. Following advice from the Company’s pre-IND meeting with the FDA, the Company intends to evaluate both drugs against multiple unrelated subtypes of influenza A in animal models and in cell culture studies.
H7N9 is a novel subtype currently circulating in China. Recent studies have indicated that it is a potential pandemic threat. H7N9 was found to be less sensitive to approved drugs than the H1N1/2009 pandemic strain. See New studies on H7N9 raise pandemic concerns | CIDRAP (http://www.cidrap.umn.edu/news-perspective/2013/07/new-studies-h7n9-raise-pandemic-concerns).
The Company has recently developed drug candidates for evaluation against the novel MERS h-CoV (“Middle East Respiratory Syndrome human Coronavirus”). The Obama administration has designated MERS as a potential threat to public health and national security, on June 4, 2013. Administration declares Mideast flu a potential public health emergency - The Hill's Healthwatch (http://thehill.com/blogs/healthwatch/public-global-health/303441-administration-declares-mideast-flu-a-potential-public-health-emergency). No drugs or vaccines are available against MERS h-CoV.
The Company has recently executed a NDA with the UK Public Health Agency that is intended to lead to a Master Service Agreement for the evaluation of FluCide against the novel A/H7N9 influenza strain as well as evaluation of the Company’s novel drug candidates against the newly emerging MERS human Coronavirus. The Company believes that independent testing of our drug candidates at these two sites should result in a dataset providing a high degree of confidence.and
NanoViricides Receives Notification that Clinical Coordinators Have Been Appointed by The European Medicine Agency to Review the Company’s Upcoming DengueCide Orphan Drug Designation Application
Mon, Jul 22, 2013 7:00 AM EDT - Excerpts:
WEST HAVEN, Conn.--(BUSINESS WIRE)-- NanoViricides, Inc. (OTC BB: NNVC) (the "Company") announced today that it has been notified that the Committee on Orphan Medicinal Products (COMP) of the European Medicine Agency (EMA) has appointed a team of clinical coordinators for the purpose of reviewing the Company’s upcoming orphan drug designation application for DengueCide™, its drug candidate for the treatment of dengue and dengue hemorrhagic fever.
COMP has appointed this team in response to the Company’s submission of a letter of intent to file an Orphan Drug Application with the European Medicines Agency (EMA) for DengueCide™. NanoViricides intends to file this application after the appropriate notice period (usually 60 days) has expired. The actual application will need to be translated into 27 different languages prior to submission.
The Company previously engaged the consulting firm Cote´ Orphan Consulting (COC), headed by Dr. Tim Cote´, to assist with the orphan drug application. The Company, in consultation with COC, has determined that its current lead DengueCide drug candidate is eligible for orphan drug status application in the European Union. The Company has recently filed an Orphan Drug Designation application for DengueCide to the US FDA.
DengueCide is a nanoviricide® that has shown very high effectiveness in an animal model of dengue virus infection. These animal studies were conducted in the laboratory of Dr. Eva Harris, Professor of Public Health and Infectious Diseases at the University of California, Berkeley. Professor Harris has developed a mouse model simulating antibody-dependent-enhancement (ADE) of dengue infection using a special laboratory mouse strain called AG129. ADE in humans is thought to lead to dengue hemorrhagic fever, and is associated with a high fatality rate. In this model, infection with a dengue virus, when the mice are left untreated, is 100% fatal. In contrast, in the same study, animals treated with NanoViricides' DengueCide achieved an unprecedented 50% survival rate.
There is currently neither an effective drug treatment nor a vaccine for dengue virus infection. Tremendous efforts have been made for dengue vaccine development but, to date, no vaccine candidate has succeeded in clinical trials towards approval.
An orphan designation for our dengue drug candidate, if granted, is expected to help the Company assign a higher priority to its dengue drug program, and undertake rapid development following the influenza drug candidates.
About Dengue and Dengue Hemorrhagic Fever
Dengue fever, a very old disease, is caused by dengue viruses. Dengue viruses occur in four different subtypes and infect humans upon being bitten by a mosquito carrying the virus. The disease can result in severe pathology even with a primary infection. However, when a person gets a second infection, with a different subtype of the virus, the disease pathology can be very severe, and can lead to hemorrhagic fever (DHF), with a high case fatality rate. Hyper-endemicity, i.e. the co-circulation of multiple serotypes, has increased the potential for DHF. Increased global temperatures are expected to continue to spread the disease into subtropical areas. In 2013, this mosquito-borne disease is one of the most important tropical infectious diseases globally, with an estimated 400 million cases of dengue fever, over one million cases of dengue hemorrhagic fever, and 50,000-100,000 deaths annually. Although the disease is endemic in many tropical parts of the world, it is considered an orphan disease in the USA and Europe. (From Clinical Microbiology Reviews).
 |
Click for full size images |
Long Term Results that Speak for Themselves
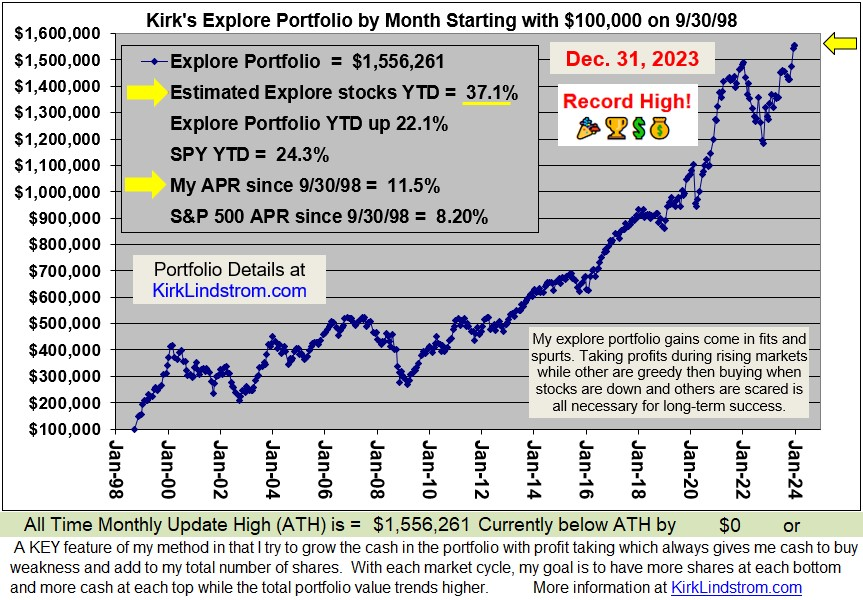
Since 9/30/98 inception, "Kirk's Newsletter Explore Portfolio" is UP 450%
vs. the S&P500 UP only 106% vs. NASDAQUP only 101% (All through 6/30/13)Since 12/31/98: 9.0% Compound Annual Return vs. 3.7% for the S&P500
(More Info, Testimonials & Portfolio Returns)
Since 9/30/98 inception, "Kirk's Newsletter Explore Portfolio" is UP 450%
vs. the S&P500 UP only 106% vs. NASDAQUP only 101% (All through 6/30/13)Since 12/31/98: 9.0% Compound Annual Return vs. 3.7% for the S&P500
(More Info, Testimonials & Portfolio Returns)
NNVC BUY ALERT!






No comments:
Post a Comment