Finisar (FNSR) Soared After Hours today on Strong Guidance for record revenues for the just completed quarter.
From Yahoo! Finance InPlay
5:07PM Finisar guides Q1 above prior guidance/consensus (FNSR) 19.13 -0.88 : Co issues upside guidance for Q1 (Jul), sees EPS of $0.30 to $0.31, excluding non-recurring items, vs. $0.24 Capital IQ Consensus Estimate, exceeding the previously estimated range of $0.22 to $0.26; sees Q1 (Jul) revs of $266 mln vs. $253.40 mln Capital IQ Consensus Estimate and compared to guidance of $245 to $260 million.
The revenue results are preliminary and subject to adjustment. However, in the absence of material adjustment, first quarter revenues will set a new record for the Company and will be the fourth consecutive quarter of sequential revenue growth. The growth in revenue came primarily from increased sales of 10G, 40G and 100G Ethernet transceivers for datacom applications. Approximately $2 million of the revenue growth over the prior quarter was from products for telecom applications.
As a result of these higher than expected revenues, a favorable product mix and increased operating leverage, the Company expects non-GAAP gross margin to be 34.5% to 35%.
stock is halted - Press Release Below
SUNNYVALE, CA--(Marketwired - Aug 6, 2013) - Finisar Corporation (NASDAQ: FNSR) today announced that, on the basis of preliminary financial results, the Company expects to report revenues of approximately $266 million for its first fiscal quarter, ended July 28, 2013, compared to guidance of $245 to $260 million that the Company provided early in the first quarter. The revenue results are preliminary and subject to adjustment. However, in the absence of material adjustment, first quarter revenues will set a new record for the Company and will be the fourth consecutive quarter of sequential revenue growth. The growth in revenue came primarily from increased sales of 10G, 40G and 100G Ethernet transceivers for datacom applications. Approximately $2 million of the revenue growth over the prior quarter was from products for telecom applications.
As a result of these higher than expected revenues, a favorable product mix and increased operating leverage, the Company expects non-GAAP gross margin to be 34.5% to 35%. This exceeds the previous guidance of approximately 33%. Non-GAAP earnings per share are expected to be $0.30 to $0.31 for the quarter, exceeding the previously estimated range of $0.22 to $0.26. A complete assessment of cost of revenues and operating expenses is not yet available but, results under GAAP are expected to include additional non-cash and infrequently occurring charges.
QUARTERLY CONFERENCE CALL
The Company expects to release its first quarter financial results after the market close on Thursday, September 5, 2013 and to discuss the first quarter results and its current business outlook during its regular quarterly conference call scheduled for Thursday, September 5, 2013, at 2:00 pm PDT (5:00 pm EDT).
Click Images to View Full Size
My Articles about FNSR on Seeking Alpha -
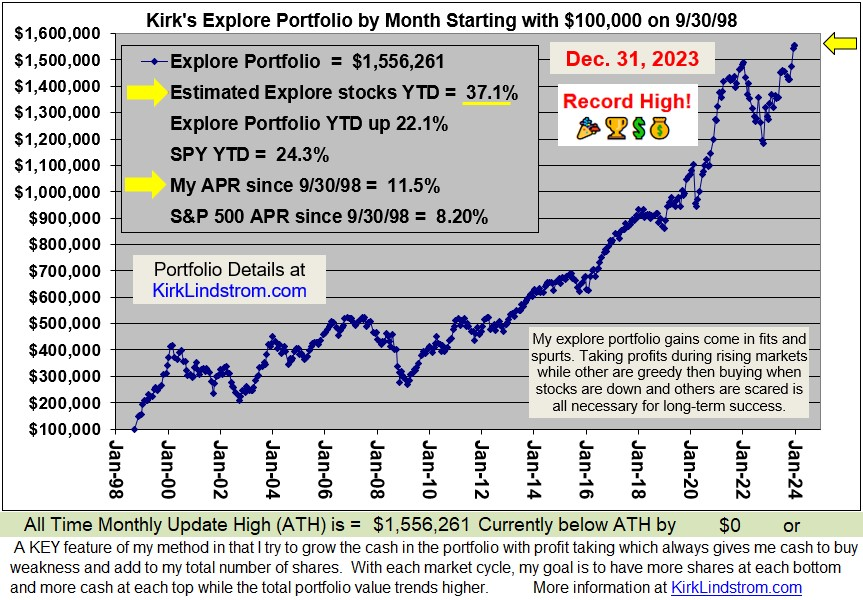
Long Term Results that Speak for ThemselvesSince 9/30/98 inception, "Kirk's Newsletter Explore Portfolio" is UP 450%vs. the S&P500 UP only 106% vs. NASDAQ UP only 101% (All through 6/30/13)Since 12/31/98: 9.0% Compound Annual Return vs. 3.7% for the S&P500
(More Info, Testimonials & Portfolio Returns)
Subscribe to my Newsletter NOW and get the August 2013 Issue for FREE! !
Your 1 year, 12 issue subscription will start with next month's issue.