As of today's close at $1.53, AGEN is up 219% YTD.
 Click chart courtesy of stockcharts.com for full size image
Click chart courtesy of stockcharts.com for full size image
HURRY! Subscribe NOW and get the June 2009 Issue of "Kirk Lindstrom's Investment Newsletter" for FREE! !
 Click chart courtesy of stockcharts.com for full size image
Click chart courtesy of stockcharts.com for full size imageHURRY! Subscribe NOW and get the June 2009 Issue of "Kirk Lindstrom's Investment Newsletter" for FREE! !
Interim survival data showed that patients with kidney cancer at intermediate risk of disease recurrence demonstrated an approximately 46% lower risk of death when treated with Oncophage® compared to the control group. Press Release
Adding to the good news, survival comes without significant "toxicities"
“Demonstration of an overall survival benefit remains the gold standard, and these interim results show that Oncophage has a real promise of improving survival in patients with earlier-stage disease for whom current prognosis remains poor,” said Dr. Wood. “Based on these results, Oncophage offers these patients a potential treatment that extends survival without significant toxicities.”About Oncophage:
In April 2008, Oncophage was approved in Russia for the adjuvant treatment of kidney cancer patients at intermediate-risk for disease recurrence. Pre-commercial launch activities are ongoing. In October 2008, Antigenics submitted a marketing authorization application to the European Medicines Agency (EMEA) requesting conditional approval for Oncophage in earlier-stage, localized renal cell carcinoma.
Derived from each individual’s tumor, Oncophage contains the ‘antigenic fingerprint’ of the patient’s particular cancer and is designed to reprogram the body’s immune system to target only cancer cells bearing this fingerprint. Oncophage is intended to leave healthy tissue unaffected and limit the debilitating side effects typically associated with traditional cancer treatments such as chemotherapy and radiation therapy. Oncophage has been studied in Phase 3 clinical trials for the treatment of kidney cancer and metastatic melanoma and is currently being investigated in a Phase 2 trial in recurrent glioma.
Oncophage received fast track and orphan drug designations from the US Food and Drug Administration (FDA) for both kidney cancer and metastatic melanoma as well as orphan drug designation from the EMEA for kidney cancer. In 2009, Oncophage also received orphan drug designations from the FDA and EMEA for glioma.
In April 2009, the World Vaccine Congress named Oncophage as the best therapeutic vaccine.
Even if you do not own the stock, you have to hope the company is successful as the treatment has the potential to work with almost all cancers.
Disclaimer: I own and cover AGEN in "Kirk Lindstrom's Investment Letter" with my last buy below the current price as of this writing. I may sell or buy more shares without notice here but I will update my subscribers of any changes.
HURRY! Subscribe NOW and get the June 2009 Issue of "Kirk Lindstrom's Investment Newsletter" for FREE! !
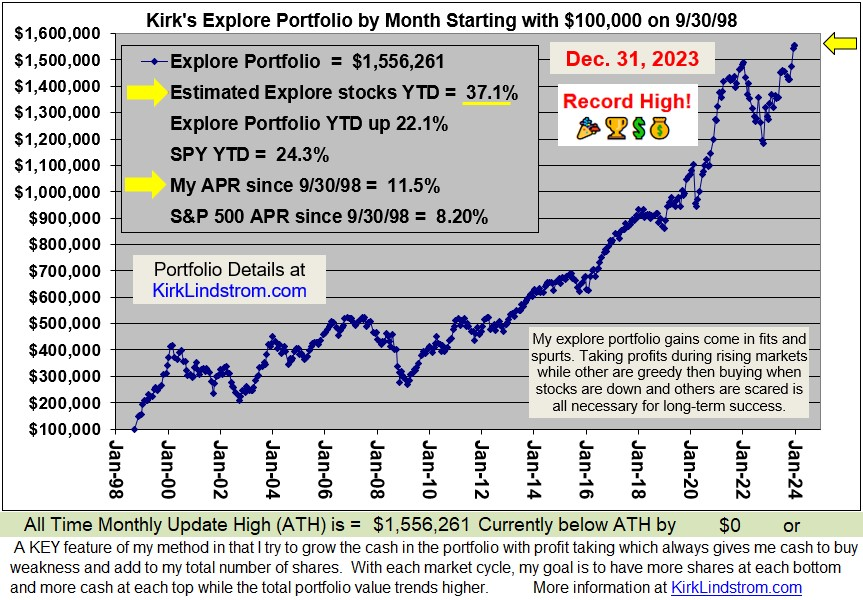
Doubled Money in a Down Market!
Since 12/31/98 "Kirk's Newsletter Explore Portfolio" is UP 113% (over a double!) vs. the S&P500 DOWN 9% vs. NASDAQ down 16% vs. Warren Buffett's Berkshire Hathaway (BRKA) up 28% (All through
As of June 4, 2009, "Kirk's Newsletter Explore Portfolio" is up 10% YTD
vs. DJIA DOWN 0.3%
(More Info & FREE Sample Issue)
vs. DJIA DOWN 0.3%
(More Info & FREE Sample Issue)



No comments:
Post a Comment